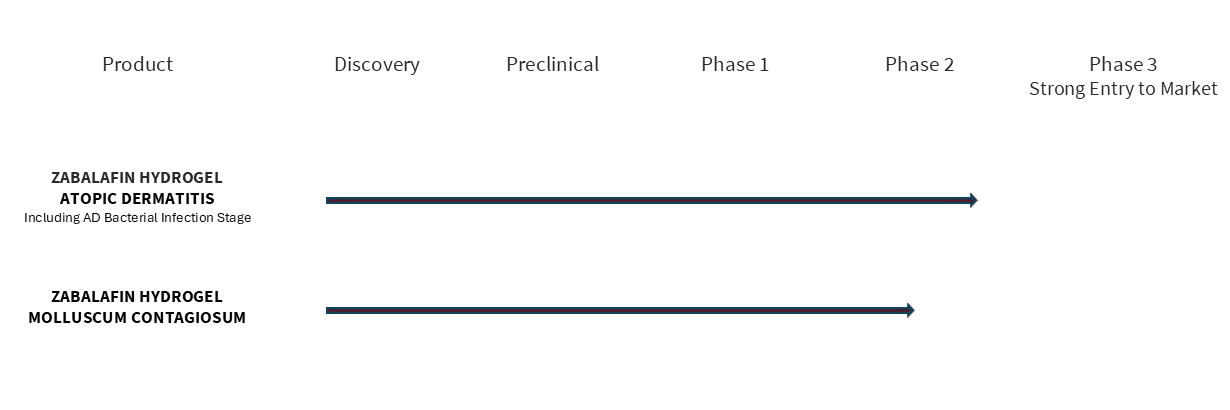
We are advancing the first Multi-Target Therapeutics® (MTT™) based on our Zabalafin Platform (AB-101 Platform) to treat and cure severe and common skin diseases. Alphyn’s Platform produces therapeutic candidates with the potential to be safer and more effective, and the possibility for faster development times and lower costs than standard drugs. Alphyn’s lead product candidate, zabalafin hydrogel (AB-101a) has completed a Phase 2a clinical trial evaluating adults and children age 2 and up with atopic dermatitis, in 2 cohorts, with and without infected AD skin. The trial met all endpoints, demonstrating significant and clinically relevant improvements in itch, quality of life, inflammation, elimination of infected AD skin and control of Staph bacteria- associated and other AD Flares with minimal side effects and excellent patient tolerability. Planning for a global Phase 2b trial is under way.
Our Vision: Complete, Effective and Safe.
A New Approach to Atopic Dermatitis.
Alphyn has developed zabalafin hydrogel to be the first complete treatment for atopic dermatitis (AD), the most common form of eczema. With multiple bioactive components, zabalafin hydrogel is expected to directly attack and eliminate the itch, bacteria component and inflammation component of AD by directly targeting the bacteria living on the AD skin that discharge toxins that make AD symptoms worse and often cause infection and prevent healing.
Zabalafin hydrogel is a non-steroidal, topical AD treatment, which is preferred by patients. It is targeted to offer patients and physicians a complete treatment for AD without the safety concerns of long-standing and new or experimental therapies. Zabalafin hydrogel’s safety, side effect and patient tolerability profile suggests it may be the first AD topical drug that can be used worry-free, long-term, and continuously.
AD is a chronic disease that affects 10% percent of the population worldwide or some 800 million people globally. Its symptoms vary by individual but often include short or prolonged periods of severe inflammation, itchiness, rashes, scaly patches, blisters, and skin infections that cover small-to-large portions of the body.

70%
Itch is severe, unbearable

55%
Inadequate disease control

5.9 Million
Lost workdays each year

160%
Increase in suicide risk
While historically viewed as exclusively an autoimmune disease, a growing body of evidence indicates bacteria living on AD skin contribute to AD, making it more severe and difficult to treat. The common bacteria Staphylococcus aureus (or Staph) naturally lives on the skin and releases toxins. Approximately 30 to 60 percent of AD patients have alpha toxins produced by the bacteria Staphylococcus aureus an its various strains1, 2 that are significantly associated with AD2. In patients with AD, who have a skin barrier dysfunction, this triggers an immune system response causing inflammation, itch, and sometimes infection—the more bacteria on the skin, the more severe the AD. Unfortunately, more than 30 percent of AD infections are caused by Methicillin-resistant Staphylococcus aureus, or MRSA, which is difficult to kill and can become life-threatening. Antibiotics are used to eliminate these bacteria, allowing the other AD drugs to be more helpful.
Many patients suffering from AD must take multiple medicines to treat their disease, and many of these therapeutics have safety considerations.
1 Wichmann K, Uter W, Weiss J, et al. Isolation of alpha-toxin-producing Staphylococcus aureus from the skin of highly sensitized adult patients with severe atopic dermatitis. Br J Dermatol. 2009;161(2):300–305.
2 Breuer K, Wittmann M, Kempe K, et al. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088–1095.


